My areas of interest include the development of soft polymeric materials for applications in bioengineering, bioelectronics, and sustainability. I have 7+ years of experience in polymer synthesis and characterization, with expertise in controlled radical polymerization, polymer functionalization, molecular characterization (NMR, GPC, FTIR, DLS, XPS, Raman spectroscopy, UV-visible spectroscopy), rheology and electrochemistry.

Next-generation soft materials for bioengineering and bioelectronics
Effect of Polymer Architecture on Gel Mechanics
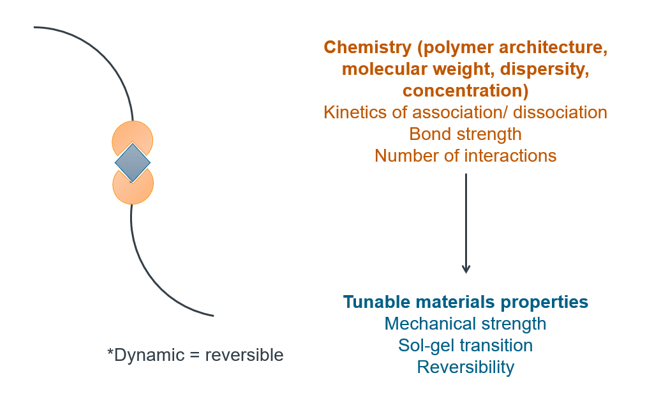
Project overview: Dynamic covalent gels are a class of polymer networks that consist of reversible, and hence dynamic, covalent bonds. These gels have found applications in wastewater remediation, as scaffolds for tissue engineering, and in drug delivery, with potential for use as templates for soft conductive gels, stimuli-responsive materials, and tissue mimics. The range of mechanics of these gels is dictated by the molecular structure of the polymer backbone, such as the polymer architecture, molecular weight, number of crosslinking points, and mechanism of crosslinking. However, the effect of the polymer architecture on the mechanics is not well understood. In this work, I am developing dynamic covalent gels with varying polymer architecture, and studying their mechanical properties under shear. The intellectual merit of this work lies in the design of new polymer backbones to elucidate the effect of gel architecture on the mechanics. The broader impacts of this work include the development of materials platforms with tunable mechanical properties, with potential for further use in self-healing gels and composites. This is an on-going project.
Thermo-reversible and conductive hydrogels based on PEDOT:PSS

Thermo-reversible PEDOT-based gels. a. Reversible gelation of TR-CPs. b. Mechanical characterization. Change in modulus as a function of temperature, and time (at 37 °C) for 3.6 wt% TR-CP in DI water, pH = 2. c. Electronic characterization of TR-CPs in PBS, pH = 4. d. Patterning of TR-CPs in pre-heated viscous alginate solution. e. s-EMG set-up with TR-CP electrodes.
Representative publication: “Thermo-reversible gelation of self-assembled conducting polymer colloids”
“Thermo-responsive and conducting polymer with a reversible sol-gel transition”, US Patent App. US20250197549A1
Project overview: Conductive polyelectrolyte complexes such as PEDOT:PSS can act as the link between the conductivity of inorganic materials (metals) and the low Young’s modulus and ionic conductivity of biological tissue. The incorporation of such conducting polyelectrolyte complexes in hydrogels is becoming increasingly favorable for applications in on-skin electrochemical recording, soft actuators and tissue engineering. Current methods of synthesizing conductive hydrogels based on PEDOT:PSS result in gels that are irreversibly crosslinked and do not adapt or respond to changes in their environment. This behavior limits their use in applications that require shape-conformability, such as wound healing or tissue engineering, in vitro cell phenotyping, reusable soft electronics and 3D bioprinting. To overcome these challenges, we developed thermo-reversible conductive polymers (TR-CPs) based on PEDOT:PSS. TR-CPs were found to be electronically and ionically conductive, tolerant to a wide range of pH and aqueous buffers and biocompatible with L929 cells. We showed that TR-CPs can be patterned into tissue-like substrates using a fine needle and demonstrated their use in bioelectronics as reusable s-EMG electrodes. The intellectual merit of this work is that we enabled the formation of soft, injectable conductive hydrogels without chemical crosslinkers (which can leach out), endogenous metabolites (which are typically either tissue- or species- specific) and blends (which cannot be used to control the microstructure). The broader impacts include wide adoption of TR-CPs for potential applications in shape-conformable electronics, injectable and patternable interfaces, and scaffolds for tissue engineering.
Thermo-responsive polyelectrolytes as proteoglycan mimics in cartilage
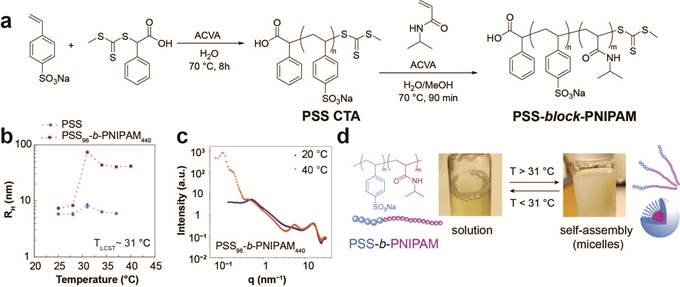
Thermo-responsive polyelectrolytes. a. Synthesis of PSS-block-PNIPAM, a thermo-responsive and conductive polyelectrolyte complex. b. Change in hydrodynamic radius as a function of temperature for PSS and PSS-b-PNIPAM. c. Change in intensity as a function of q, as observed by small angle x-ray scattering (SAXS). d. Schematic for micelle formation in PSS-b-PNIPAM.
Representative publication: “Enhanced diffusion and retention of proteoglycan replacements in cartilage through thermo-responsive polyelectrolytes”, manuscript under revision
“Application of synthetic polyelectrolytes for restoration of glycosaminoglycan or proteoglycan function in soft tissue”, PCT International Patent Application No. PCT/US24/54137.
Project overview: Musculoskeletal degenerative disorders such as osteoarthritis (OA) occur due to rapid degradation of the proteoglycans and glycosaminoglycans (GAG) in knee joints, resulting in loss of fixed charge density, reduction in structural integrity and collapse of the cartilage tissue. Current treatments for OA utilize GAG replacements such as chondroitin sulfate, which are ineffective due to their lack of retention in degraded cartilage tissue. To overcome this challenge, in collaboration with Prof. Charles Dhong at UD, we utilized polyelectrolytes such as polystyrene sulfonate (PSS) as GAG replacements, which showed improved retention due to their compact morphology and charged polymeric backbone. However, PSS retention in cartilage is limited due to its small particle size and the diffusion kinetics of PSS result in a non-homogenous distribution of polymer chains in the tissue, mostly limited to the edges. Thus, to improve retention and ensure even distribution of polyelectrolytes throughout the cartilage, I developed thermo-responsive block copolymers (t-BCPs) of PSS with poly (N-isopropylacrylamide) (PNIPAM), a thermo-responsive polymer which undergoes a hydrophilic-hydrophobic transition (LCST) at 31-35 °C (close to body temperature). My collaborators from the Dhong Lab found that found that t-BCPs displayed significantly improved retention and even distribution throughout tissue over 5 days. The intellectual merit of this work is in demonstrating, for the first time, that thermo-responsive polyelectrolytes have excellent potential for use as GAG replacements. The broader impacts of the work imply human health outcomes in advancing the development of long-term treatments for osteoarthritis.